BS EN ISO 7899-2 pdf free download
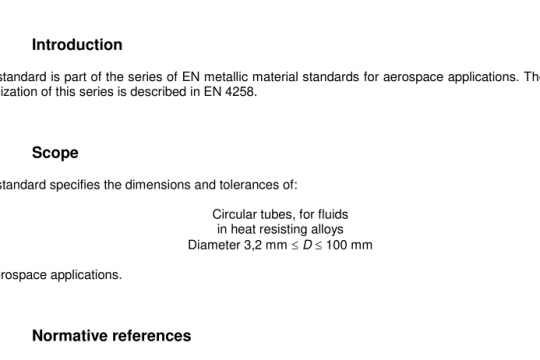
BS EN ISO 7899-2 pdf free download. Water quality – Detection and enumeration of intestinal enterococci – Part 2: Membrane filtration method.
3 Terms and definitions For the purposes of this part of ISO 7899, the terms and definitions given in ISO/IEC Guide 2 and the following apply. 3.1 intestinal enterococci bacteria which are able to reduce 2,3,5-triphenyltetrazolium chloride to formazan and to hydrolyse aesculin at 44 °C on the media (6.3.1 and 6.3.2) specified in this part of ISO 7899 NOTE See also annex A. 4 Principle 4.1 Filtration, incubation and enumeration The enumeration of intestinal enterococci is based on filtration of a specified volume of water sample through a membrane filter with a pore size (0,45 μm) sufficient to retain the bacteria. The filter is placed on a solid selective medium containing sodium azide (to suppress the growth of Gram-negative bacteria) and 2,3,5-triphenyltetrazolium chloride, a colourless dye, that is reduced to red formazan by intestinal enterococci. Typical colonies are raised, with a red, maroon or pink colour, either in the centre of the colony or throughout. 4.2 Confirmation If typical colonies are observed, a confirmation step is necessary, by transfer of the membrane, with all the colonies, onto bile- aesculin-azide agar, preheated at 44 °C. Intestinal enterococci hydrolyse aesculin on this medium in 2 h. The end-product, 6,7-dihydroxycoumarin, combines with iron(II) ions to give a tan-coloured to black compound which diffuses into the medium. 5 Apparatus Except for disposable glassware which is delivered sterile, glassware shall be sterilized in accordance with ISO 8199. Usual microbiological laboratory equipment and particularly: 5.1 Membrane filtration apparatus, according to ISO 8199. 5.2 Sterile membrane filters, with a nominal pore size of 0,45 μm. The quality of membrane filters may vary from brand to brand or even from batch to batch. It is therefore advisable to check the quality on a regular basis, in accordance with IsO 7704. 5.3 Incubator, capable of being maintained at 36 °C士2 °C. 5.4 Incubator, capable of being maintained at 44。C士0,5 °C. 5.5 Autoclave, capable of being maintained at 121 °C士3 °C. 6 Culture media and reagents 6.1 Basic materials WARNING一The selective media described in this part of ISO 7899 contain sodium azide. As this substance is highly toxic and mutagenic, precautions shall be taken to avoid contact with it, especially by the inhalation of fine dust during the preparation of commercially available dehydrated complete media. Azide-containing media should not be mixed with strong inorganic acids, as toxic hydrogen azide (HN3) may be produced. Solutions containing azide can also form explosive compounds when in contact with metal pipework, for example from sinks. Azides can be decomposed safely by the addition of an excess of a saturated nitrite solution. For uniformity of results, in the preparation of media, either use a dehydrated complete medium or use constituents of uniform quality and chemicals of recognized analytical grade. Sodium azide deteriorates with time so that dehydrated media have a limited shelf-life. NOTE Use of chemicals of another quality is possible provided they are shown to be of equal performance in the test. 6.2 Distilled water or water of equivalent purity, in accordance with ISO 3696, Grade 1. 8 Procedure 8.1 Preparation of the sample Prepare the sample, filter and inoculate on isolation media in accordance with the instructions given in ISO 8199 and ISO 6887-1. Start the examination preferably immediately after taking the samples. If the samples are kept at ambient temperatures, the examination shall begin within 6 h after taking the sample. Under exceptional circumstances, it is permissible for the samples to be kept at 5 °C土3 °C for up to 24 h prior to examination. If sample dilutions are necessary, prepare these dilutions in accordance with ISO 8199. BS EN ISO 7899-2 pdf download.